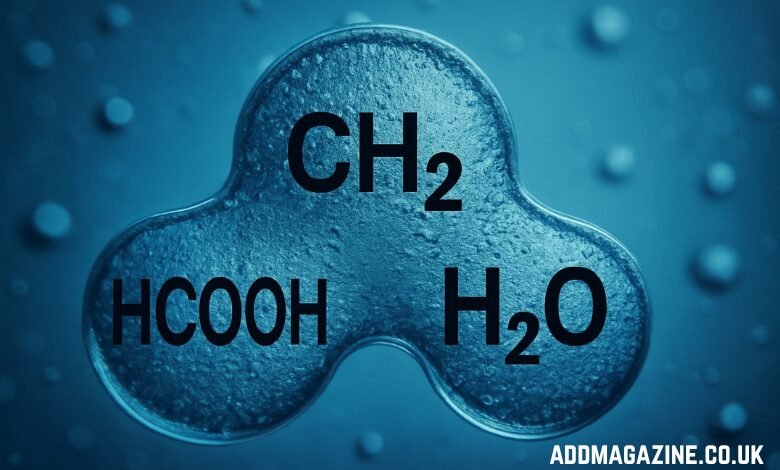
In organic chemistry, the study of molecular structures and reactions often unveils fundamental principles that drive industrial and academic advancements. One such combination that holds significance in various chemical reactions and processes is the trio of molecules: HCOOH, CH2, and H2O. These molecules, which represent formic acid (HCOOH), a methylene group (CH2), and water (H2O), are commonly encountered in both laboratory research and industrial applications. Though these molecules seem simple at first glance, their interactions provide critical insights into the world of chemistry and its wide-ranging applications. In this article, we will break down the structure, significance, and applications of each molecule in this trio, helping to clarify their relevance in organic chemistry.
Introduction to HCOOH, CH2, and H2O
The combination of HCOOH, CH2, and H2O holds an important place in the study of organic chemistry. Formic acid (HCOOH), the methylene group (CH2), and water (H2O) are essential building blocks that participate in various chemical reactions that are integral to industrial processes, biological functions, and laboratory studies. These molecules each serve distinct roles, and when combined, they help drive numerous reactions, particularly in the synthesis of other compounds and materials. Whether in academic research or industrial applications, the understanding of these molecules and their behavior is critical for chemists, engineers, and biologists alike.
Definition of HCOOH, CH2, and H2O
Formic Acid (HCOOH)
Formic acid, represented by the molecular formula HCOOH, is one of the simplest carboxylic acids. It consists of a single carbon atom bonded to both a hydroxyl group (-OH) and a carbonyl group (C=O). Formic acid is found naturally in the venom of ants, as well as in the stings of other insects. Industrially, it plays a significant role as a chemical intermediate and a preservative in various applications. It is also utilized in agriculture, textiles, and leather production, among others. Formic acid is known for its strong acidic properties, which make it useful in catalytic processes and as a reducing agent in chemical reactions.
Methylene Group (CH2)
The methylene group (CH2) consists of a single carbon atom bonded to two hydrogen atoms. It is one of the simplest organic groups and plays a pivotal role in a wide array of chemical reactions. Methylene groups are often involved in the formation of more complex organic structures through processes such as polymerization and condensation. The presence of the methylene group is common in many hydrocarbons, making it a fundamental part of organic chemistry. Its ability to bond with various functional groups makes it a key component in creating diverse chemical compounds.
Water (H2O)
Water, represented by the molecular formula H2O, is perhaps the most well-known and abundant compound on Earth. Its role in organic chemistry is vast and indispensable. Water serves as a solvent in many chemical reactions, facilitates the formation of hydrogen bonds, and is involved in the process of hydration and hydrolysis. It is involved in countless reactions, both biological and chemical, and its properties are fundamental to life as we know it. The polarity and hydrogen-bonding ability of water make it a vital molecule in organic chemistry, particularly in biological systems where it is involved in processes such as protein folding, enzyme activity, and metabolic reactions.
Breaking Down the Components: Understanding Each Molecule
Now that we have defined each molecule in the trio, let’s break down what each represents in the context of organic chemistry.
Formic Acid (HCOOH)
Formic acid, being one of the simplest carboxylic acids, has a wide range of applications. Its molecular structure consists of a carbonyl group (C=O) bonded to a hydroxyl group (-OH), making it a prototypical carboxylic acid. In terms of chemical reactions, formic acid is often used as a reducing agent, particularly in the synthesis of other organic compounds.
Formic acid can participate in esterification reactions, where it reacts with alcohols to form esters. These esters have varied uses, including as solvents, fragrances, and in the production of plastics and resins. Moreover, formic acid is involved in the catalytic process for the production of biodiesel, a renewable energy source, by transesterification of fats and oils.
In addition to industrial uses, formic acid also plays a crucial role in biological systems. For example, it is part of the metabolic processes in humans and animals and is produced as a byproduct of anaerobic respiration in certain microorganisms.
Methylene Group (CH2)
The methylene group is a fundamental building block in organic chemistry, acting as a bridge between two other functional groups in a molecule. It can participate in a variety of reactions, such as alkylation, polymerization, and substitution reactions.
In many cases, the methylene group is involved in the creation of larger molecules, including polymers and complex organic compounds. For instance, methylene groups are key components in the formation of polyolefins, which are widely used in the manufacturing of plastics and synthetic fibers.
The methylene group also plays a vital role in biochemistry. It is often found in the backbone of many biomolecules, including lipids, proteins, and nucleic acids, where it helps maintain molecular structure and function.
Water (H2O)
Water is arguably the most essential molecule in organic chemistry, serving as the solvent for countless chemical reactions. Its polarity allows it to dissolve a wide range of polar substances, making it indispensable in both synthetic and biological chemistry.
One of water’s most critical roles in organic chemistry is in hydrolysis reactions, where water molecules break down larger molecules into smaller components. This process is essential in digestion and cellular metabolism. In synthetic chemistry, water is often used to facilitate the formation of bonds between other molecules, such as in condensation reactions where water is a byproduct.
Additionally, water plays a role in stabilizing the structures of large organic molecules, such as proteins and nucleic acids. The hydrogen bonds between water molecules and other polar groups in these biomolecules help maintain their three-dimensional structure, which is crucial for their biological function.
Applications of HCOOH, CH2, and H2O in Organic Chemistry
The combination of formic acid (HCOOH), the methylene group (CH2), and water (H2O) is fundamental in various chemical processes. These molecules are involved in reactions that are essential in many industrial and biological contexts.
- Catalytic Processes: Formic acid’s role as a reducing agent and its ability to participate in esterification reactions makes it crucial in catalytic processes. It is used in the production of biodiesel and other industrial chemicals, where it helps in the transformation of raw materials into useful products.
- Polymerization: The methylene group’s presence in many organic molecules makes it essential in the synthesis of polymers. Polymers such as polyethylene, polystyrene, and PVC are formed through the linkage of methylene groups, which provide the backbone for these long-chain molecules.
- Hydrolysis and Condensation Reactions: Water plays a crucial role in both hydrolysis and condensation reactions. In the hydrolysis of esters, for instance, water breaks down complex molecules into their constituent parts, a process that is critical in digestion and cellular metabolism. In condensation reactions, water is a byproduct, but its presence helps drive the reaction forward, facilitating the formation of more complex molecules.
- Biological Processes: In biological systems, these three molecules are essential for life. Formic acid is produced in anaerobic respiration, the methylene group is part of the molecular structure of various biomolecules, and water is the solvent for many biochemical reactions, including enzyme activity and protein folding.
Significance of HCOOH, CH2, and H2O in Organic Chemistry
The combination of formic acid (HCOOH), methylene group (CH2), and water (H2O) is significant in organic chemistry because of the fundamental roles each component plays in a wide array of chemical reactions and processes. These molecules are not only essential in industrial chemistry but also hold importance in biological systems, highlighting their versatility and relevance.
- Formic Acid (HCOOH):
- Versatile Reducing Agent: Formic acid acts as a reducing agent, especially in the synthesis of other chemicals. Its ability to donate electrons makes it useful in reduction reactions in industrial processes.
- Precursor to Other Chemicals: It is used in the synthesis of various important chemicals like formate esters and methanol.
- Agricultural Applications: Formic acid is often used as a preservative and antimicrobial agent in animal feed and is also employed in pest control.
- Methylene Group (CH2):
- Building Block in Polymerization: The methylene group is a key structural unit in many organic compounds. It participates in polymerization reactions, leading to the formation of polymers used in plastics, textiles, and synthetic fibers.
- Structural Foundation for Organic Molecules: It acts as a backbone in a variety of organic molecules, contributing to the stability and functionality of larger chemical structures.
- Water (H2O):
- Universal Solvent: Water’s role as a solvent is crucial in both biological systems and chemical reactions. It is involved in numerous reactions, such as hydrolysis, where it helps break down complex molecules into simpler forms.
- Hydrogen Bonding: Water molecules exhibit strong hydrogen bonding, which is important in stabilizing organic structures, especially proteins and nucleic acids in biological systems.
Properties of HCOOH, CH2, and H2O
Each of the components in this trio—formic acid, the methylene group, and water—has distinct properties that influence how they participate in chemical reactions.
Formic Acid (HCOOH)
- Molecular Structure: Formic acid is a simple carboxylic acid with the structure HCOOH. It consists of a carbonyl group (C=O) attached to a hydroxyl group (–OH). This structure makes formic acid an acidic compound capable of donating protons (H⁺).
- Physical Properties: It is a colorless, pungent liquid with a strong acidic odor. It has a boiling point of 100.8°C and is highly soluble in water. Formic acid is highly corrosive and should be handled with care.
- Reactivity: Formic acid can participate in esterification reactions, where it reacts with alcohols to form esters. It also acts as a reducing agent, particularly in organic reactions where electron transfer is necessary.
Methylene Group (CH2)
- Structure: The methylene group consists of a single carbon atom bonded to two hydrogen atoms. It is often found as part of larger molecular structures.
- Physical Properties: As a functional group, CH2 is not a standalone molecule but a part of larger molecules, such as in methylene bridges in polymers. It is highly reactive, especially when it interacts with other functional groups in organic synthesis.
- Reactivity: The methylene group is involved in various reactions such as substitution, polymerization, and condensation. It is often a key player in the formation of larger organic structures, including plastics and synthetic fibers.
Water (H2O)
- Structure: Water is a polar molecule with a bent shape, where the oxygen atom is bonded to two hydrogen atoms. The oxygen atom is more electronegative, leading to a partial negative charge on the oxygen and a partial positive charge on the hydrogens.
- Physical Properties: Water is colorless, odorless, and tasteless. It has a high specific heat capacity and surface tension, which are key to its role in both biological and chemical processes. It freezes at 0°C and boils at 100°C under standard atmospheric conditions.
- Reactivity: Water is involved in a range of reactions, especially hydrolysis (where it breaks down molecules) and condensation reactions (where water is a byproduct). It also forms hydrogen bonds, contributing to the stability of biomolecules.
Reactions Involving HCOOH, CH2, and H2O
Esterification Reaction
Esterification is a key organic reaction where an alcohol reacts with a carboxylic acid, such as formic acid (HCOOH), to form an ester and water. This reaction is commonly catalyzed by an acid or a base.
- Mechanism: In esterification, formic acid reacts with an alcohol (e.g., ethanol) to form an ester (ethyl formate) and water. The reaction involves the nucleophilic attack of the alcohol’s hydroxyl group on the carbonyl carbon of the formic acid, releasing water in the process. Example: HCOOH+CH3CH2OH→CH3CH2OCOH+H2O\text{HCOOH} + \text{CH}_3\text{CH}_2\text{OH} \rightarrow \text{CH}_3\text{CH}_2\text{OCOH} + \text{H}_2\text{O}HCOOH+CH3CH2OH→CH3CH2OCOH+H2O In this reaction, ethanol (CH3CH2OH) reacts with formic acid (HCOOH) to form ethyl formate (CH3CH2OCOH) and water.
- Significance: Esterification reactions are important in the production of fragrances, plastics, solvents, and other industrial chemicals. In this reaction, water is both a byproduct and a driving force for equilibrium, and its removal can drive the reaction forward.
Hydrolysis Reaction
Hydrolysis is a chemical reaction in which water breaks down a compound into two smaller molecules. In the context of formic acid (HCOOH) and methylene groups (CH2), hydrolysis can break down larger organic compounds.
- Mechanism: In ester hydrolysis, water breaks the ester bond, separating the ester into its parent alcohol and carboxylic acid. For example, the hydrolysis of ethyl formate (CH3CH2OCOH) would produce ethanol (CH3CH2OH) and formic acid (HCOOH). Example: CH3CH2OCOH+H2O→HCOOH+CH3CH2OH\text{CH}_3\text{CH}_2\text{OCOH} + \text{H}_2\text{O} \rightarrow \text{HCOOH} + \text{CH}_3\text{CH}_2\text{OH}CH3CH2OCOH+H2O→HCOOH+CH3CH2OH This reaction is a reverse esterification process where water adds back to the ester bond, breaking it into formic acid and ethanol.
- Significance: Hydrolysis is essential in biological systems for digesting complex molecules like proteins and polysaccharides. In industry, hydrolysis is used in the breakdown of polymers to produce monomers, facilitating the recycling of materials.
Laboratory Context: Applications of HCOOH, CH2, and H2O
In the laboratory, the combination of formic acid (HCOOH), methylene groups (CH2), and water (H2O) plays a critical role in various synthetic and analytical processes.
- Synthesis of Organic Compounds: Formic acid is often used as a reagent in organic synthesis to introduce carboxyl groups into molecules or reduce other compounds. Methylene groups act as bridges in organic compounds, especially in the creation of polymer materials.
- Polymer Chemistry: The methylene group is a crucial part of polymerization reactions, where it helps form the backbone of polymers. In laboratory settings, methylene groups are often used in the synthesis of nylon, polyester, and other plastics.
- Hydrolysis in Analytical Chemistry: Water is often used in the hydrolysis of esters, which is a key step in many analytical procedures. In the laboratory, hydrolysis reactions help break down complex compounds, facilitating the analysis of their components.
- Formic Acid in Analytical Chemistry: Formic acid is commonly used in high-performance liquid chromatography (HPLC) and mass spectrometry (MS) as a mobile phase modifier or solvent, aiding in the analysis of complex biological and chemical samples.
Conclusion
The combination of formic acid (HCOOH), the methylene group (CH2), and water (H2O) represents a set of fundamental molecules that play a significant role in organic chemistry. Each of these molecules has distinct properties and applications, yet they often interact in chemical reactions that are crucial for both industrial processes and biological systems. Whether it’s the role of formic acid as a reducing agent, the involvement of the methylene group in polymerization, or the essential function of water as a solvent and participant in hydrolysis and condensation reactions, these molecules help drive the chemical reactions that form the foundation of life and industry.
Understanding the importance of HCOOH, CH2, and H2O is vital for chemists, engineers, and biologists alike. Their widespread presence and involvement in various chemical processes make them integral to the world of organic chemistry.
FAQs
- What is the role of formic acid (HCOOH) in organic chemistry?
- Formic acid acts as a reducing agent and participates in esterification and catalytic reactions, contributing to industrial processes.
- How does the methylene group (CH2) participate in chemical reactions?
- The methylene group is a key component in polymerization, substitution reactions, and the formation of complex organic molecules.
- Why is water (H2O) important in organic chemistry?
- Water acts as a solvent, facilitates hydrolysis and condensation reactions, and stabilizes biomolecular structures through hydrogen bonding.
- What is esterification, and how does it involve HCOOH, CH2, and H2O?
- Esterification is a reaction where formic acid (HCOOH) and an alcohol form an ester, producing water (H2O) as a byproduct.
- How does hydrolysis work with the molecules HCOOH, CH2, and H2O?
- Hydrolysis uses water (H2O) to break down esters like ethyl formate, releasing formic acid (HCOOH) and alcohol.